– Remco Driessens, Manager Key Account Team at MAG45
6 November 2025
6 November 2025

More than 75 years ago, MAG45 was founded from within one of Europe’s largest med tech manufacturers. What began as a warehouse has grown into a global partnership model that supports med tech and high-tech leaders around the world. Today, that same collaborative spirit drives how MAG45 helps this customer build compliant and future-ready supply chains. A partnership that is characterized by long-term trust, open communication, and a shared drive to create a resilient supply chain.
In the medical technology sector, compliance is ever evolving. Every component must be traceable, every supplier verifiable, and every shipment auditable.
“Compliance and traceability are key for this customer. Because they ship worldwide, every screw and cable must meet the standards in the Americas, Europe, and Asia.”
MAG45 helps ensure that standard by managing supplier documentation such as Certificates of Conformity (CoC), REACH and RoHS declarations, and material certificates for new and legacy parts. MAG45’s experts sit side-by-side with the customer’s teams, solving problems together and building systems that make compliance better every year. All information is gathered, verified, and uploaded into the customer’s systems, ensuring each part can be traced back to its origin. “We regularly check supplier compliance and documentation to make sure nothing slips through the cracks,” Remco explains.
This diligence enables customers to introduce new products faster and within the high product compliance requirements. “Without the right documentation, you simply can’t enter regulated markets,” says Remco. “Our job is to make sure everything is ready so they can focus on innovation instead of paperwork.”
In the med tech industry, full traceability is essential for meeting FDA and international regulations. “Everything needs to be proven,” Remco explains. “Including the raw material composition and the place of manufacturing. If that information is not available or missing, importing the goods into the regions becomes very challenging.”
By combining procurement expertise with compliance management, MAG45 maintains what Remco calls “the paper trail behind every product.” The company’s structured process centralizes certificates, origin labels, and safety data sheets, enabling customers to demonstrate evidence over every part of their tail supply chain.
“When one of our customers launches a new medical system, we make sure every small component is certified and traceable from day one.”
The result is greater reliability and fewer disruptions. Every new product launch is a joint effort. From the first prototype to the point where it is taken into regular production, MAG45 and the customer’s teams work hand in hand to ensure every component meets the highest standards.
Global trade regulations and import tariffs are changing faster than ever, creating new challenges for manufacturers. “If you design in Europe and sell in Europe, the Americas and Asia, you’re facing complex rules that evolve constantly,” says Remco. “That’s where we step in to help keep supply chains moving smoothly.”
“It’s not just about avoiding tariffs. Local-for-local strategies help improve efficiency, reduce transport emissions, and support sustainable growth.”
Many manufacturers are now exploring local-for-local production, where products are sourced and built within the same region to reduce complexity and risk. With operations in Europe, Asia, and North America, MAG45 is ready to help customers adapt their supply chains to these new realities. By building regional supply networks that meet local compliance and sustainability requirements, the company helps customers become more agile and resilient in a fragmented global market.
Over the years, MAG45 has developed deep expertise in compliance and traceability by working closely with its customers. MAG45 didn’t start as compliance experts, but with hiring compliance experts and managing daily execution, a strong foundation had been built. Now that experience is used to support other med tech and high tech companies.”
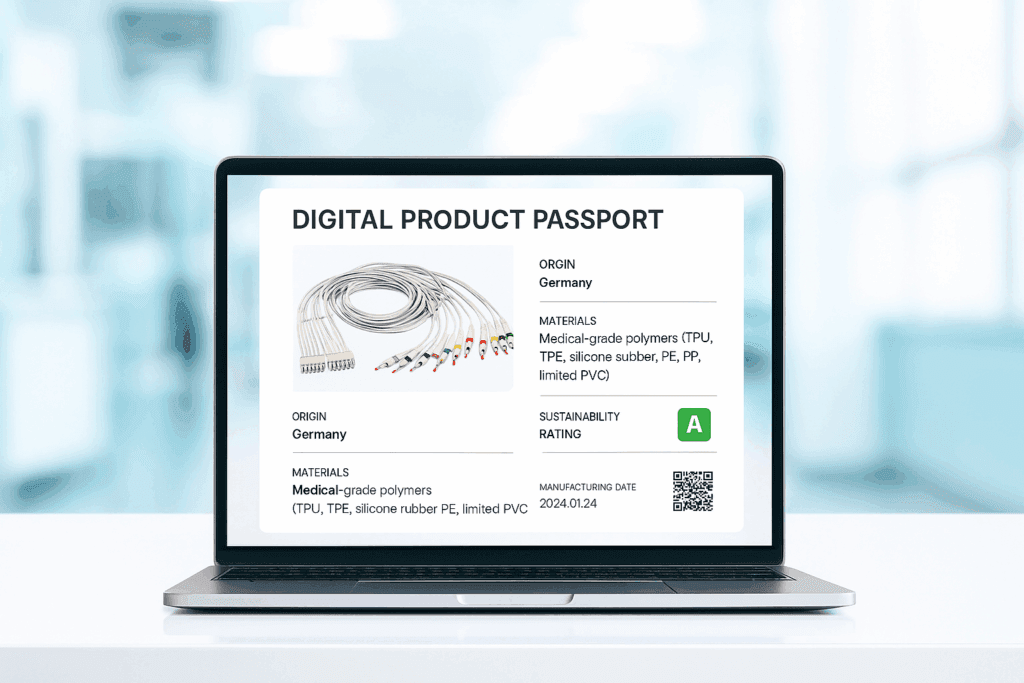
Looking ahead, MAG45 MAG45 has developed digital product passports; comprehensive digital records that track materials, sourcing, and sustainability for each item.
“Imagine every product having a passport that shows what it’s made of, where it originates, and how it was produced. That’s where the world might be going, and we’re preparing for it.”
Today, MAG45 not only helps customers maintain a tail supply chain that is transparent, reliable, and ready for change. MAG45’s partnerships go far beyond logistics. It also includes compliance, sustainability, and co-engineering to meet customer demand, driving added value and cost savings.
“It’s about trust and continuous improvement,” Remco concludes. “Our customers know we’ll do whatever it takes to make sure they can deliver safely, efficiently, and in line with every regulation.”
For MAG45, future-proofing isn’t just a promise. It’s the foundation of how the company works with every partner to strengthen the supply chain of tomorrow.